\”
The fight to slow global warming mostly has focused upon weaning humans away from burning fossil fuels that emit carbon dioxide and contribute to the greenhouse effect. There\’s also been a lot of effort to find ways to capture CO2 out of the air and put it someplace where it can\’t do any harm. Of course, the perfect solution would be to accomplish both things at once. What if you could take CO2 out of the atmosphere and employ it a cleaner energy source, reducing the need to burn fossil fuel?
Scientists from Vanderbilt and George Washington universities may have found a way to do just that. In a paper published today in the American Chemical Society journal ACS Central Science, they describe a process to extract carbon from atmospheric CO2, and then use it to make carbon nanotubes. The nanotubes would then be used to replace the graphite electrodes in lithium-ion batteries for electric-powered cars.
In theory, we could create not just carbon-neutral, but carbon-negative, electric cars that store power and counteract past environmental damage.
"Given their better performance, projected low cost, and capability to remove a greenhouse gas, it is likely that carbon nanotube battery-equipped cars will become the norm," says one of the scientists, GWU chemistry professor Stuart Licht, via email.
In a press release announcing the development, Vanderbilt assistant professor of mechanical engineering Cary Pint said, "Imagine a world where every new electric vehicle or grid-scale battery installation would not only enable us to overcome the environmental sins of our past, says but also provide a step toward a sustainable future for our children."
How Would This Work?
The new battery-making method uses a process developed by Licht and his GWU colleagues for capturing carbon and using it to make carbon nanofibers, which can be bundled to create nanotubes. That process involves deploying concentrated solar energy to create a molten bath of chemicals that reaches 1,380 degrees F (749 degrees C). When air is added to the cell, the carbon dioxide dissolves when subjected to the heat and direct current from nickel and steel electrodes.
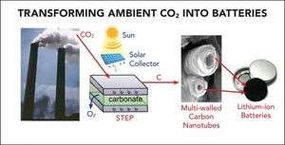

As the gas breaks down, the carbon molecules cling to the electrodes and build up into nanofibers. After Licht and his team published their work in 2015, it promised to be a potential game-changer. Not only did it provide a method for creating carbon nanofiber that was cheaper than previous methods, but it also offered a way to extract huge amounts of carbon dioxide from the atmosphere.
When that development was announced last year, Licht told HowStuffWorks that he envisioned building an array of giant C02-to-nanofiber plants the size of cities in sparsely populated places such as the Australian Outback, and the Sahara and Mojave deserts.
Since it\’s super-strong as well as lightweight, carbon nanofiber has been touted as the material of the future for everything from skyscraper girders to aircraft fuselages. But carbon nanotubes fashioned from such fibers also are pretty great for making batteries, because their large surface area enables them to store more of a charge than other forms of carbon. Back in 2010, Massachusetts Institute of Technology researchers created an experimental battery with carbon nanotubes that had a third more capacity than a conventional lithium-ion battery, and 10 times the power output.
The GW and Vanderbilt researchers report that a lithium-ion battery with carbon nanotube electrodes also performs slightly better than a conventional lithium-ion battery, and that the boost is amplified when the battery is charged quickly.
When they used the nanotubes to replace graphite electrodes in a sodium-ion battery, another type of storage, they got an even bigger improvement — about 3.5 times the performance. Both types of batteries equipped with carbon nanotubes successfully endured 10 weeks of continuous charging and discharging with no signs of fatigue.
Practical Applications for Advances
According to Licht, putting batteries with carbon nanotubes in cars will "provide greenhouse gas emission-free alternatives to today\’s industrial and transportation fossil fuel processes."
Gina Coplon-Newfield, director of the Sierra Club\’s Electric Vehicles Initiative, said that while she hasn\’t yet seen the specifics of the Vanderbilt-GWU breakthrough, "it sounds really intriguing." "Generally, we\’re very encouraged by what we see happening in battery technology these days," Coplon-Newfield says. "That\’s both in terms of the technological advances, and the costs coming down."
The process for using atmospheric carbon dioxide to make batteries wouldn\’t have to just be used for electric cars. It also could be put to use to make lithium-ion batteries for electronic devices, and also in much larger batteries that could be used to store electricity generated by solar panels and wind turbines.
Having that sort of storage is crucial for developing future "smart" electrical grids that rely upon smaller, decentralized sources of electricity, instead of being dependent upon huge coal-burning plants.
Now That\’s Interesting
The use of batteries as storage devices for energy goes back hundreds, if not thousands, of years.











